Schedule 2 Narcotics List
The Drug Enforcement Administration (DEA) has its decision: It will keep marijuana in the same legal, regulatory category as heroin — schedule 1. To many people, this is outrageous. Obviously, marijuana is nowhere as dangerous as heroin.
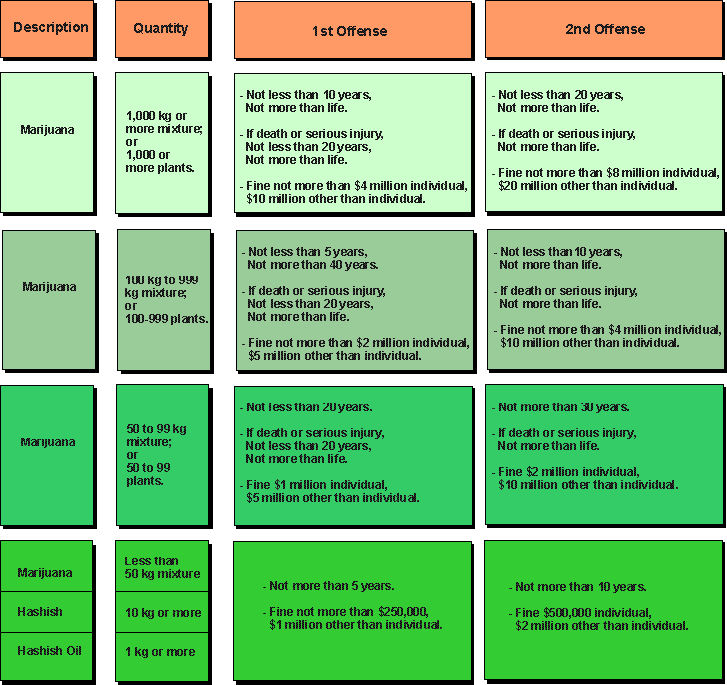
Schedule 2 Narcotics List Pdf
And it's not more dangerous than schedule 2 drugs like cocaine and meth. So why the hell is pot schedule 1? But the classification doesn't mean that the federal government thinks of marijuana and heroin as equally dangerous drugs. The schedule reflects a more complicated system — one that accounts for a drug's medical value as much as a drug's potential for abuse.

A harsh schedule also does not mean a drug is totally illegal., while guided by the scheduling system, often take other factors into account. For pot, they do — leaving it as one of the illicit drugs at the federal level, even though it's schedule 1. And opioid painkillers, as one example, are schedule 2 but legal for medical purposes. Still, a drug's schedule is an important policy guide. A stricter schedule lets the DEA more stringently limit access to a drug and its supply, which can make a drug more difficult to research — as has happened for marijuana, limiting researchers' ability to study the drug for its medical value. ( As a result, advocacy and medical groups have that pot's schedule is out of step with the available scientific evidence.) So what is the scheduling system, how does it work, and what would it take to reschedule a drug? Here's what you need to know. Hakikat negara secara umum.
Schedule II/IIN Controlled Substances (2/2N) Substances in this schedule have a high potential for abuse which may lead to severe psychological or physical dependence. Examples of Schedule II narcotics include: hydromorphone (Dilaudid®), methadone (Dolophine®), meperidine (Demerol®), oxycodone (OxyContin®, Percocet®), and fentanyl. Schedules of controlled substances. In accordance with sections 3 and 4 of the act (35 P. § § 780-103 and 780-104), this section lists all controlled substances. Section 4 of the act (35 P. § 780-104) designates specific substances for inclusion under the five schedules.
How does the US classify illicit drugs like marijuana? Stan Honda/AFP via Getty Images Under the, the federal government — which has largely relegated the regulation of drugs to the Drug Enforcement Administration (DEA) — puts each drug into a classification, known as a schedule, based on its medical value and potential for abuse. To initiate a schedule, the DEA first asks if a drug can be abused. If the answer is yes, then it's put on a schedule. If no, the drug is left out.
After that, the drug's medical value and relative potential for abuse are evaluated to decide where on the scale it lands. Congress did not clearly define abuse under the Controlled Substances Act The two big issues, then, are a drug's potential for abuse and its medical value. Congress did not clearly define abuse under the Controlled Substances Act. But for federal agencies responsible for classifying drugs, abuse is when individuals take a substance recreationally and develop personal health hazards or pose other risks to society as a whole. To find medical value, a drug must have large-scale clinical trials to back it up — similar to what the Food and Drug Administration (FDA) would expect from any other drug entering the market. Schedule 1 drugs have no medical value and high potential for abuse, while schedule 2 through 5 substances all have some medical value but differ in ranking depending on their potential for abuse (from high to low). Some examples of the drugs that are on each schedule: • Schedule 1: marijuana, heroin, LSD, ecstasy, and magic mushrooms • Schedule 2: cocaine, meth, oxycodone, Adderall, Ritalin, and Vicodin • Schedule 3: Tylenol with codeine, ketamine, anabolic steroids, and testosterone • Schedule 4: Xanax, Soma, Darvocet, Valium, and Ambien • Schedule 5: Robitussin AC, Lomotil, Motofen, Lyrica, and Parepectolin In general, schedule 1 and 2 drugs have the most regulatory restrictions on research, supply, and access, and schedule 5 drugs have the least.